Fantastic ceramic
23 Aug 2016 by Evoluted New Media
From joint replacements to microfluidics – additive manufacturing, or 3D printing, promises so much for the biomedical industry.
From joint replacements to microfluidics – additive manufacturing, or 3D printing, promises so much for the biomedical industry. Here, Dr Tassilo Moritz takes us through the materials science of this rapidly growing field.
Suspension-based additive manufacturing (AM) methods offer new possibilities for producing personalised or application-oriented ceramic components for manifold applications in many industrial branches – not least for biomedical products.
AM allows the production of components layer-by-layer. Thus, a complexity in geometry can be attained over conventional manufacturing technologies. The advantages of these additive technologies can be further increased by combining them or adding other shaping technologies. Examples shown in this article are: a combination of lithography-based ceramic manufacturing (LCM) with aerosol-jet printing and LCM with freeze foaming for manufacturing a functionalised micro fluidic device and a patient specific bone replacement component, respectively.
AM technologies can be classified according to the state of the material that is used – powder, liquid and solid
According to the American Society of the International Association for Testing and Materials (ASTM), AM is a process of joining material to make objects from 3D model data, usually layer upon layer¹. The additive manufacturing of polymers is state-of-the-art and in the field of metals the processing of more and more materials has been accomplished. For processing ceramic materials however, the technical application of AM technologies has been limited. Nevertheless, ceramic materials have been studied in additive manufacturing processes ab initio with the development of different AM technologies over the last 25 years²,³.
AM technologies can be classified according to the state of the material that is used? – powder, liquid and solid. It is also possible to classify the AM technologies according the dimensional order? – point, line or plane. All established AM technologies have been tested for ceramic materials. 3D powder bed printing is the most commonly used AM process for powder materials. Typical application of this technique focuses on the production of porous ceramic components because of the low compaction of the powder layers; the green density (a measure determining the amount of shrinkage required to densify a ceramic) is too low to reach high density after sintering. However, for a number of applications high densities are not required, a good example of this is bioactive scaffold structures. For instance, scaffold structures based on calcium phosphates?-¹? or porous glass-ceramics¹¹ have been produced by 3D powder bed printing. Selective laser sintering (SLS) was tested for a number of ceramic materials, too¹²-¹?. These methods are so-called powder-based AM techniques, because the components are built up starting from a loosely packed powder bed.
Additive manufacturing has the potential to advance microreaction technology because the channel routing can be carried out in the most efficient way, i.e. channels can be designed offering excellent mixing results over the shortest distances.
The main disadvantage of these methods is to be seen in the fact that a powder bed always also goes along with packing inhomogeneities, which cannot be eliminated, either by binder infiltration or by interaction with a laser beam. Nor is subsequent sintering sufficient for attaining a completely densified ceramic body. For this problem, suspension-based AM technologies offer a solution because particle distribution is much more homogeneous in suspensions, pastes, inks or thermoplastic feedstocks than in a dry powder compact. Thus after sintering, a much improved density and ceramic structure can be attained, providing dense components with increased mechanical properties.
Furthermore, the direct printing of suspensions has proved a promising way to produce dense ceramic materials by additive manufacturing¹?,¹?. Conventional stereo lithography (STL) for example, has been applied for aluminium oxide, zirconia, silicon nitride and silica as well as for zirconia toughened alumina (ZTA) ceramics²?-²³. In this STL process, a photopolymerisable ceramic suspension is cured by an UV-laser. Based on the principal approach of using photopolymerisable binders in the ceramic suspension or paste, specific AM techniques have been developed for the production of ceramic green bodies. For example, a robocasting process uses UV curable inks with high Al2O3 loading²?. Binders cured under visible blue light were applied in a direct light processing (DLP) process, which allows the production of dense alumina parts with complex shapes²?.
Two examples will be used here to show the advantageous combination of either two additive manufacturing technologies or an AM method with a foaming technique. The first example shows a novel route to produce patient-specific bone replacement material. The second example is a microfluidic device, which may serve as micromixer or microreactor for gases or liquids. Additive manufacturing has the potential to advance microreaction technology because the channel routing can be carried out in the most efficient way, i.e. channels can be designed offering excellent mixing results over the shortest distances. Inner wall openings and spoilers may be manufactured which could never have been attained by conventional shaping techniques. Another advantage of AM – no coverage of channel structures is needed anymore, because micro reactors can be made dense. Furthermore, fluidic ports for inlet and outlet of fluidic media can be printed simultaneously. Currently, fluidic ports have to be joined by brazing – often a weak point for leakage. Beside applications in the chemical industry or pharmaceuticals, micro fluidic devices may be of interest as sterilisable micro mixers for patient specific mixtures of drugs – or as micro labs. By adding a second component to the microfluidic device – like a conductive path – functionalised components with sensor functions or heatable micro reactors can be produced.
A bone to pick… At the largest scale, bone macrostructure consists of two different configurations which differ in the level of porosity: cortical (dense, 5-10% porosity) and cancellous (spongy, 50–90% porosity) bone²?. Combining two different shaping methods – freeze foaming and lithography-based ceramic manufacturing (LCM) – allows us to attain a similar structure and to provide the component with a patient-specific outer shape comparable to the naturally grown bone. The LCM-technology is a slurry-based process, where ceramic powder is homogenously dispersed in a photo-curable monomer system and selectively polymerised through mask exposure. This will initially give the so-called green part. During the thermal post-treatment, the organic matrix is removed via pyrolysis and the particles are densified during sintering to give the dense ceramic body. These two process steps are typical also of conventional ceramic forming technologies. A layer of the suspension is then applied by the rotation of the vat in combination with a static wiper blade. The bottom of the vat is transparent; thus, a light source can illuminate the suspension from below through the vat. The projected image is generated via a digital micro-mirror device (DMD). The resolution of the DMD is 1920 x 1080 pixels.
Using a dedicated optical system the resolution in x/y-plane is adjusted to 40µm and a minimum wall thickness of 120µm can be achieved. The sintered surfaces show a roughness (Ra) between 0.4 and 1µm. The building platform is above the vat and moves the z-axis upwards during the fabrication process. The LCM machine enables the production of alumina and zirconia components with a relative density up to 99.4% and 99.0%, respectively. For manufacturing the cortical bone structure, a photo-curable suspension basing on hydroxyapatite powder has been developed. For that purpose the powder with a D50-value of 2.64µm and a Brunauer–Emmett–Teller (BET) surface of 70.0m2/g was heat treated at 900°C for 2 hours. The photosensitive polymeric suspension was mixed in a speed mixer. A solid content of 37 vol.% hydroxyapatite was attained by adding a dispersing aid.
For combining the freeze foam as model of a trabecular bone structure with the “cortical” bone structure made by LCM, the shrinkage of both structures during sintering must be adjusted very precisely for avoidance of any cracks in the components. For that purpose, testing structures have been designed – cylindrical hollow parts with pimples inside for improved adhesion of the foam in the structure and hollow hemispheres. These structures were filled with aqueous suspensions of the same hydroxyapatite powder with a solid content of 41 vol.%, which had been found to be adequate for an adjusted shrinking behaviour. Afterwards, the structures were put into a freeze drying chamber.
Commonly, foaming techniques are based on the thermal decomposition of, often environmentally harmful, organic volatile pore formers or, in the case of replica techniques, even whole polymer scaffolds. In contrast, freeze foaming is a direct foaming technique starting from an aqueous suspension of the ceramic powder. The freeze foaming process results from a reduction of the ambient pressure as the suspension is exposed in a freeze dryer. The applied vacuum causes an inflation of the foam by vapour and entrapped air acting as inflation media. Ongoing pressure reduction simultaneously reduced the equilibrium temperature of the foam. When reaching the triple point of water, the foam freezes suddenly producing a stiff structure. Therefore, the pore formers are mostly rising water vapour, air and the sublimation of frozen water.
[caption id="attachment_54934" align="alignnone" width="620"] Figure 1. : Bone replacement testing parts after sintering - the “cortical” component was made by LCM, the “trabecular” structure by freeze foaming.[/caption]
Figure 1. : Bone replacement testing parts after sintering - the “cortical” component was made by LCM, the “trabecular” structure by freeze foaming.[/caption]
After removal of the frozen suspension media by a subsequent sublimation process, the ceramic green foam is sintered. As a result, ceramic foam has been generated showing a bimodal pore size distribution – large pores of 400–800µm in the foam cells and 40-60nm in the ligaments of the foam. Such freeze foam made of hydroxyapatite has been proven to show advantageous ingrowth conditions for mesenchymal human stem cells²7. After sublimation drying, the parts had been debindered and co-sintered (at 1350°C) to one bio composite as can be seen in Figure 1. From the computed tomography images (Figure 2) it becomes visible that the “trabecular” foam structure and the “cortical” LCM structure form a uniform component with a firm material fit devoid of cracks in the interface region.
[caption id="attachment_54935" align="alignnone" width="620"]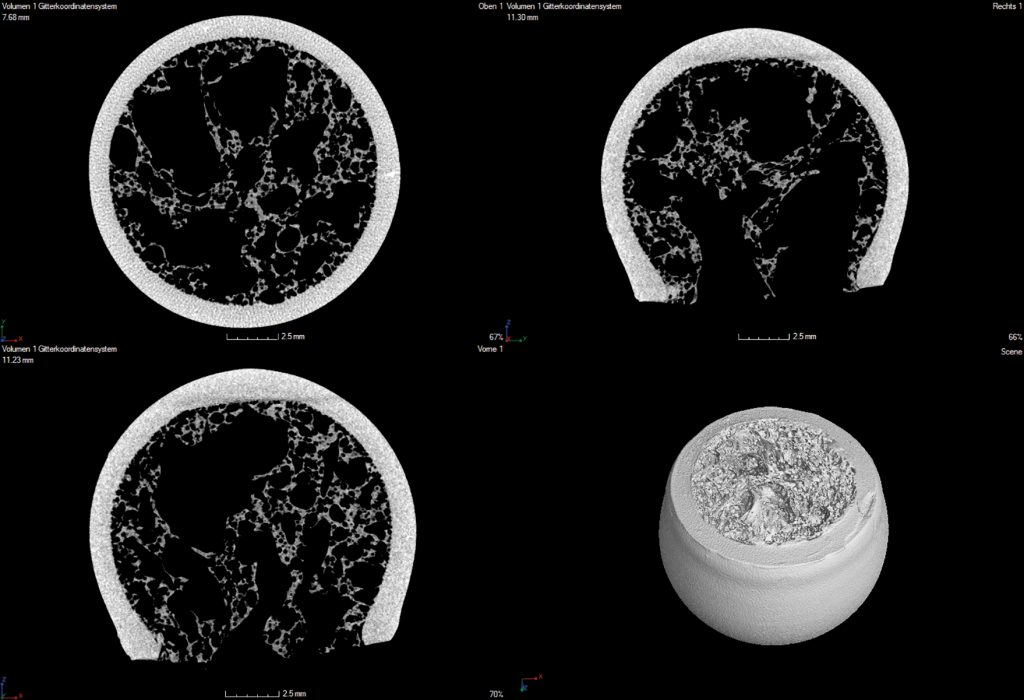 Figure 2. Computed tomography images of bone replacement testing parts showing defect-free interfaces between “trabecular and cortical” structure.[/caption]
Figure 2. Computed tomography images of bone replacement testing parts showing defect-free interfaces between “trabecular and cortical” structure.[/caption]
Micro machines Using the LCM technique, a demonstrator for a micro fluidic device has been manufactured using a commercially available alumina suspension. It was designed to combine several features, which were not feasible before using additive manufacturing. The component has two fluidic ports, one inlet and one outlet which are simultaneously manufactured in one processing step without any additional joining steps. The demonstrator shows a change in the cross-section from circular fluidic ports to quadratic body. Moreover, the inner fluidic channel structure is very complex and ensures an intensive mixing of different fluids. The manufactured green part was debindered and sintered to full density (99.4%). In a subsequent step, a second AM method was used. A meandered heating structure and electrical contacts were applied by aerosol-jetting. The heating conductor consists of 42vol.% ruthenium oxide (RuO2) and a special glass. For aerosol-jetting, both components were applied as a suspension. Then, the printed structures were sintered again at 850°C for 10min. The heatable demonstrator component is shown in Figure 3.
[caption id="attachment_54936" align="alignnone" width="620"] Figure 3. Functionalised micro fluidic device, made by LCM (ceramic body) and aerosol-jetting (heating conductor).[/caption]
Figure 3. Functionalised micro fluidic device, made by LCM (ceramic body) and aerosol-jetting (heating conductor).[/caption]
Ceramic additive manufacturing allows the production of novel components of very high, so far unfeasible, complexity for customised and personalised products. The degree of functionalisation can be further increased by combining AM techniques with conventional shaping routes.
Author: Dr Tassilo Moritz is group leader of the working group “Shaping” at the Fraunhofer Institute for Ceramic Technologies and System IKTS in Dresden.
Contributing authors: Matthias Ahlhelm, Uwe Scheithauer, Eric Schwarzer, Markus Eberstein
References:1 ASTM-Standard F2792 -12a: Standard Terminology for Additive Manufacturing Technologies. March 1, 2012, ASTM International Distributed under ASTM license by Beuth publisher. 2 Lakshminarayan, U.; Ogrydiziak, S.; Marcus, H.L.: Selective lasersintering of ceramic materials, Proceedings of Solid Free-Form Symposium (1990) 16-26 3 Lauder, A.; Cima, M. J.; Sachs, E.; Fan, T.: Three dimensional printing: surface finish and microstructure of rapid prototyped components, Materials Research Society Symposium Proceedings, 249, 331-336, (1992) 4 Chartier, T.; Badev, A.: Rapid Prototyping of Ceramics. In: Handbook of Advanced Ceramics Elsevier, Oxford, UK, 2013 5 Travitzky, N.; Bonet, A.; Dermeik, B.; Fey, T.; Filbert-Demut, I.; Schlier, L-; Schlordt, T.; Greil, P.: Additive Manufacturing of ceramic-based materials, Advanced Engineering Materials, 16, 729-754, (2014) 6 Gbureck, U.; Hoelzel, T.; Biermann,I.; Barralet, J.; Grover, L.M.: Preparation of tricalcium phosphate/calcium pyrophosphate structures via rapid prototyping, J. Mater. Sci.: Mater. Med., 19, 1559-1563, (2008) 7 Seitz, H.; Rieder, W.; Irsen, S.; Leukers, B.; Tille, C.: Three-dimensional printing of porous ceramic scaffolds for bone tissue engineering, Biomed. Mater. Res., Part B: Appl. Biomater., 74B, 782-788, (2005) 8 Khalyfa, A.; Meyer, W.; Schnabelrauch, M.; Vogt, S.; Richter, H.-J.: Manufacturing of biocompatible ceramic bone substitutes by 3D-printing, cfi/ Ber. DKG, 83, 23-26, (2006) 9 Deisinger, U.; Irlinger, F.; Pelzer, R.; Ziegler, G.: D-printing of HA-scaffolds for the application as bone substitute material, cfi/ Ber. DKG, 83, 75-78, (2006). 10 Dombrowski, F.; Caso, P.W.G.; Laschke, M.W.; Klein, M.; Guenster, J.; Berger, G.: 3-D printed bioactive bone replacement scaffolds of alkaline substituted ortho-phosphates containing meta- and di-phosphates, Key Engineering Materials, 529-530, 138-142, (2013) 11 Zocca, A; Gomes, C.M.; Bernardo, E.; Müller, R.; Günster, J.; Colombo, P.: LAS glass–ceramic scaffolds by three-dimensional printing, J. Europ. Ceram. Soc., 33, 1525-1533, (2013) 12 Lenk, R.; Nagy, A.; Richter, H.-J.; Techel, A.: Material development for laser sintering of silicon carbide. cfi/ Ber. DKG, 83, 41-43, (2006) 13 Regenfuss, P.; Ebert, R.; Exner, H.: Laser Micro Sintering - a versatile instrument for the generation of microparts, Laser Technik Journal, 4, 26-31, (2007) 14 Hagedorn, Y.-C.; Wilkes, J.; Meiners, W.; Wissenbach, K.; Poprawe. R.: Net shaped high performance oxide ceramic parts by selective laser melting, Phys. Proced., 5, 587–594, (2010) 15 Wu, Y.; Du, J.; Choy, K.-L.; Hench, L.L.: Laser densification of alumina powder beds generated using aerosol spray deposition, J. Europ. Ceram. Soc., 27, 4727-4735, (2007) 16 Goodridge, R.D.; Lorrison, J.C.; Dalgarno, K.W.; Wood, D.J.: Comparison of direct and indirect selective laser sintering of porous apatite mullite glass ceramics, Glass Technology, 45, 94-96, (2004) 17 Sadeghian, Z.; Heinrich, J. G.; Moztarzadeh, F.: Direct Laser Sintering of Hydroxyapatite Implants by Layerwise Slurry Deposition (LSD), cfi/Ber. DKG 82, E1-E5, (2004) 18 Cappi, B.; Oezkol, E.; Ebert, J.; Telle, R.: Direct inkjet printing of Si3N4: Characterization of ink, green bodies, and microstructure, J. Europ. Ceram. Soc., 28, 2625-2628, (2008) 19 Ebert, J; Özkol, E.; Zeichner, A.; Uibel, K.; Weiss, Ö.; Koops, U.; Telle, R.; Fischer, H.: Direct Iinkjet printing of dental prostheses made of zirconia. J. Dent. Res., 88, 673-676, (2009) 20 Pham-Gia, K.; Rossner, W.; Wessler, B.; Schäfer, M.; Schwarz, M.: Rapid Prototyping of high-density alumina ceramics using stereolithography, cfi/ Ber. DKG, 83, 36-40, (2006) 21 Chartier, T.; Duterte, C.; Delhote, N.; Baillargeat, D.; Verdeyme, S.; Delage, C.; Chaput, C.J.: Fabrication of millimeter wave components via ceramic stereo- and microstereolithography processes. J. Am. Ceram. Soc., 91, 2469–2474, (2008) 22 Griffith, M.L.; Halloran, J. W.: Freeform fabrication of ceramics via stereolithography. J. Am. Ceram. Soc., 79, 2601-2608, (1996) 23 Licciulli, A.; Corcione, C.E.; Greco A.; Amicarelli, V.; Maffezzoli, A.: Laser stereolithography of ZrO2 toughened Al2O3, J. Europ. Ceram. Soc., 25, 1581-1589, (2005) 24 de Hazan, Y.; Thänert, M.; Trunec, M.; Misak, J.: Robotic deposition of 3d nanocomposite and ceramic fiber architectures via UV curable colloidal inks, J. Europ. Ceram. Soc., 32, 1187-1198, (2012) 25 Felzmann, R.; Gruber, S.; Mitteramskogler, G.; Tesavibul, P.; Boccaccini, A.R.; Liska, R.; Stampfl, J.: Lithography-based additive manufacturing of cellular ceramic structures, Adv. Eng. Mater., 14, 1052-1058, (2012) 26 Motealeh, A.: Effect of Coating on the Mechanical Behaviour and Bioactivity of Robocast Bioceramic Scaffolds. Doctoral Thesis, Universidad de Extremadura, Spain, 2015 27 Ahlhelm, M.; Günther, P.; Scheithauer, U.; Schwarzer, E.; Günther, A.; Slawik, T.; Moritz, T.; Michaelis, A.: Innovative and novel manufacturing methods of ceramics and metal-ceramic composites for biomedical applications, Journal of the European Ceramic Society (2016), Online First, 6 pp., ISSN: 0955-2219, DOI: 10.1016/j.jeurceramsoc.2015.12.020









