Smashing the accelerator science skills gap
16 Mar 2016 by Evoluted New Media
The emerging field of accelerator science promises so much for so many sectors – but do we have the skills to keep up?
The emerging field of accelerator science promises so much for so many sectors – but do we have the skills to keep up?
The most familiar use of accelerators in medicine is perhaps the use of X-rays for imaging and radiotherapy. However accelerators are also used to produce isotopes for diagnosis and therapy, and more recently accelerator-based light and neutron sources have been used to elucidate the molecular structure of proteins. Accelerator science is a fascinating sector of physics and, despite its wide ranging application across health and industry; it is still an emerging field with the potential for major breakthroughs within a narrow timeframe. The need for advances, particularly in the area of proton beam therapy, is becoming increasingly urgent.
The UK government, among others, is to invest in two new proton beam therapy centres – one at the Christie Hospital in Manchester, the other at UCL Hospital in London – which are set to open in 2018 at a cost of over £250 million. Proton beam therapy is known to have many benefits over conventional radiotherapy – which uses high-energy photons or X-rays – for specific cancer types and results have been impressive, yet control of the proton beam is known to be sub-optimal and much of the success so far has been as a direct result of highly skilled staff.
[caption id="attachment_52422" align="alignnone" width="350"]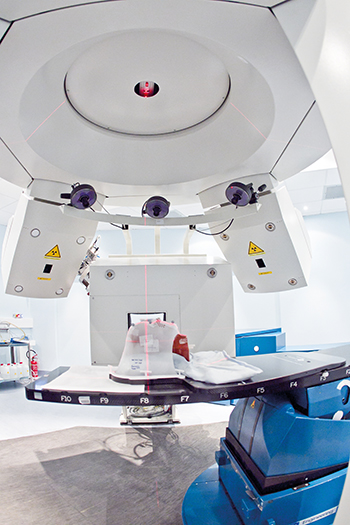 Inside of one of the three National Center of Oncology Hadrontherapy treatment rooms. Credit: CNAO[/caption]
Inside of one of the three National Center of Oncology Hadrontherapy treatment rooms. Credit: CNAO[/caption]
There is a need for better instrumentation, greater understanding of the underlying principles and more people with the skills to operate and maintain these facilities. To address this need a novel approach is being taken to fast-track research in this area. A training network began in February called Optimising Medical Accelerators. The network’s research programme has been defined with the input of partners from across universities, research centres and private companies at the leading edge of this technology to ensure it reflects the major challenges affecting the discipline. Professor Carsten P. Welsch of the University of Liverpool’s Department of Physics, based at the Cockcroft Institute, is coordinating the OMA initiative. He explains that accelerator training at this level cannot be delivered effectively by a single institute or one country: “Within OMA we are developing a medical accelerator community. The 15 research Fellows will be focused on areas of clear need will have access to a cross-sector environment for state-of-the-art research and will have opportunities to meet potential end-users. They will build a network of contacts that will be useful to them at all stages of their careers.”
Radiation therapy is a highly interdisciplinary science, and it is important to understand what is happening at a cellular and molecular level and how the energy can best be applied to achieve the medical goals.
The most common form of radiotherapy is the use of high-energy photons or X-rays, produced by an electron beam impacting a heavy-metal target. This form of radiotherapy has been successful in improving clinical outcomes. This compact instrument can be located on a gantry and rotated around the patient so that beams can hit the tumour from different directions, concentrating the dose. The higher the dose, the greater the probability of controlling the tumour; however X-ray dose deposition follows an exponential decay which means that treating a deep-seated tumour involves significant entrance and exit doses to healthy tissue around the tumour. As a result, the use of X-rays is limited when the tumour is close to sensitive organs and for use in children; as they are at higher risk of secondary cancers. Control and shaping of the dose is where proton-beam therapy has particular benefits over X-rays.
Protons are heavy charged particles that penetrate tissue for a short but precise distance and deposit most of their energy at the end of the beam so the target cancer is destroyed but the healthy tissue is spared. This remarkable phenomenon is called the ‘Bragg peak’. Dr Andrzej Kacperek is Head of the National Eye Proton Therapy Centre at the Clatterbridge Cancer Centre (CCC) NHS Foundation Trust, one of only a dozen centres in the world to offer ocular proton beam therapy. He explains the challenge: “The degree of precision is unique to proton beams. We can control how deep the beam goes so it can be used to treat a tumour on the iris for example, or one at the back of the eye. Also, as protons scatter very little the beam has sharp edges, which makes it possible to follow the outline of the tumour and protect the optic nerve. We can deliver a uniform dose by modulating the Bragg peak across the tumour depth.”However, the Douglas Cyclotron at CCC was built for a neutron therapy trial with a clinical proton beam facility built as a later addition. Thus Dr Kacperek’s team has had to develop many of its own tools to control and calibrate the monitoring instruments. In particular, a range of quality assurance (QA) procedures are carried out each day before treatment starts. The majority of this time is spent verifying the Bragg peak and prescribed patient depth dose curves. These energy QA measurements take significant time to set up and adjust for different energies.
[caption id="attachment_52423" align="alignnone" width="132"] HIT – Interior View of the HIT Linear Accelerator Developed at GSI, length 10 meters. Credit: G.Otto[/caption]
HIT – Interior View of the HIT Linear Accelerator Developed at GSI, length 10 meters. Credit: G.Otto[/caption]
It is this type of clinical challenge that will be addressed by OMA. Researchers at the Cockcroft Institute are already working with the CCC, as Professor Welsch explains: “For many years we have worked in collaboration with CCC which has generously provided us with access to their treatment beamline and allowed us to use it to take measurements. We have carried out beam transport optimisation studies, diagnostics developments and Monte Carlo simulations into dose delivery scenarios.” For example measuring the delivered dose is problematic. Charged particle beams interact with the patient’s tissue, depositing their kinetic energy though many collisions and delivering the highest energy transfer at the end of their path. Currently the dose rate is determined in a clinical environment by an interception method, but this degrades both the beam profile and its energy spread, interfering with the treatment.
Professor Welsch explains: “We are looking at non-invasive ways to measure the beam to provide quality assurance. For this we are looking at the beam halo, which is created by natural scattering of protons when the beam passes through the air to reach the patient’s eye. In particular, we are developing an online beam monitor that will give medical doctors detailed information about the treatment beam during the treatment itself. This will include the beam position, profile and intensity and hence the dose delivered to the patient. If coupled to treatment planning software and other imaging diagnostics, this would provide a complete overview and add benefit to the treatment.”
Ensuring that the beam-delivery system can accurately locate the target and define its shape is vital, and effective imaging is a major challenge, with different techniques applied at different phases of the treatment cycle.
Traditional treatment planning requires multiple patient CT images to build up an effective diagnostic image, before the start of treatment and between fractions to allow changes in the tumour volume to be monitored. However, with proton therapy the dose is very localised which requires greater imaging resolution than can be achieved by traditional CT imaging. In addition, X-ray CT images do not provide information on the proton-specific absorption characteristics of tissue surrounding the treatment volume.
An alternative is to use protons for imaging: selecting an energy where the protons do not stop within the body but instead pass through to be detected.
Using the same proton beam for both imaging and treatment would ensure that the patient does not have to be moved between imaging and treatment and the anatomical information acquired from imaging does not have to be adjusted. A research stream within OMA is to develop a conceptual proton Computed Tomography (pCT) system consisting of a series of tracking layers upstream and downstream of the patient, with some method of measuring the final energy of the diagnostic protons. It is thought that individual proton energy measurements at the 1% level are essential for a proton imaging system and the project would adapt existing calorimetry technology to provide the precise measurements required. A clinical facility would then again be used to fully characterise the performance of the detector. Additional problems arise for imaging when the patient breathes during treatment and the organs move, as the beam would need to follow this movement. Several different techniques are being considered and will also be further developed within OMA. A promising example is the camera technology pioneered by German company VIALUX that is able to determine 3D movement of objects and monitor how a patient’s lung is moving.
A major cost involved with the installation of the proton therapy or heavy ion beams such as carbon or oxygen is the cost of the infrastructure. Shrinking the machine will therefore lower the costs and make them more accessible for smaller clinical centres. For example, although gantries for both proton and heavy ion beams exist and have been built they present certain challenges. Professor Welsch explains: “A gantry for heavy ion beams is a very complex infrastructure. The Heidelberg Ion Therapy Centre, for example, features a 670 tonne gantry that can be moved around the patient, but it takes up a room the same size as a multi-story building. “It is a very sophisticated beam delivery system and I think the only one of its kind at the moment. Within OMA we will be looking both at ways to reduce the size of the accelerator and also to improve the design of the gantries.” One research stream is directed at determining a detailed beam-optical design and outline magnet design for a combined superconducting gantry and booster linac system. This will include an evaluation of different options.
This research stream will also provide opportunities for Fellows to contribute to the design and implementation of the research beamline at the Christie Hospital; a chance to be involved in implementation of a structure for testing a high-gradient proton linac and access to advice and support from CERN and TERA.This type of interdisciplinary training is exactly what Professor Welsch is seeking to provide through OMA. “OMA is at the interface between life sciences, physics and engineering. The interdisciplinary training programme that it will pioneer is unique. We are aiming to support the Fellows becoming experts in accelerator based cancer therapy, to enable them to push further developments and fully exploit these advances for the benefit of patients. We hope that this integrated approach to research and training will inspire other programmes and build research communities in other areas of need.”
About OMA
The Optimisation of Medical Accelerators Project (OMA) is a new training network set up by the University of Liverpool from the Cockcroft Institute together with a consortium of European institutions to achieve three key scientific and technological objectives:- Develop novel beam imaging and diagnostics systems.
- Enhance treatment optimisation.
- Facilitate R&D into design and optimisation to ensure optimum patient treatment along with maximum efficiency.
OMA Research Themes OMA Fellows will be involved in these exciting projects at partner institutions: 1. University of Liverpool - Halo-Dose correlation in a medical accelerator. 2. ASI - A versatile high-speed radiation detection platform. 3. CERN - Improvements on FLUKA for medical applications. 4. CNAO - Tumour tracking in particle therapy, light ion therapy software for data exchange. 5. CSIC - Application of high gradient RF technology for hadron therapy accelerators. 6. GSI - High-energy proton theranostics. 7. IBA - Imaging solutions for a novel prompt gamma camera. 8. LMU - Advanced Monte Carlo and imaging methods. 9. MedAustron - Treatment facility optimisation studies. 10. PSI - RF-based measurement of ultra-low charges. 11. UCL - Calorimeter for proton therapy and radiography. 12. University of Manchester - Gantry design for linac-boosted protons. 13. University of Seville / CAN - Radiobiological effectiveness of protons. 14. VIALUX - New encoding methodologies for ultra-fast 3D surface scanning.





