The bioluminescence advantage
13 Sep 2011 by Evoluted New Media
Although scientists draw upon a range of photon-emitting chemistries for various experimental purposes, bioluminescence has become widely preferred for quantitative bioanalysis. Keith Wood discusses the advantages that bioluminescence brings to assay methodologies
Although scientists draw upon a range of photon-emitting chemistries for various experimental purposes, bioluminescence has become widely preferred for quantitative bioanalysis. Keith Wood discusses the advantages that bioluminescence brings to assay methodologies Of the many assay technologies used in life science research, photon emission chemistries are among the most popular due to their inherently high sensitivity and simplicity. These chemistries are categorised according to how the energy for photon generation is acquired. Fluorescence, the most widely used chemistry, is also called photoluminescence because it relies on photons as the energy source. Other examples include chemiluminescence, which relies on chemical energy; radioluminescence, which relies on radioactivity (e.g. scintillants); and electroluminescence, which relies on electricity.
Bioluminescence is a form of chemiluminescence where the photon emission is based on natural biochemistry, such as the enzymatic reactions found in luminous fireflies, jellyfish, bacteria and other organisms. Bioluminescent chemistries have become increasingly popular in bioanalytical methods because they can deliver 10- to 1,000-fold higher sensitivity than fluorescence assays. This increased sensitivity can substantially improve assay performance when applied to complex biological samples1.
Fluorescence can be relatively bright since excitation photons may be introduced to a sample at very high rates, for example, by using high-intensity lasers. However, this high influx of photons can also raise background levels due to two factors: the capacity of the photodetector to discriminate between excitation and emission photons, and interference from other weak fluorophores present within the samples. It is important to consider that the number of photons introduced into a fluorescent assay is enormous relative to those produced by the reporters. In contrast, although enzyme catalysis typically yields less light intensity due to slower excitation rates, the background for bioluminescence is extremely low since no photons are introduced into the sample.
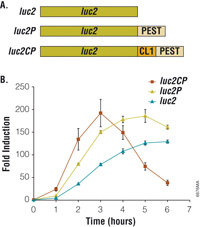 |
| Figure 1: Bioluminescent reporter assay of the beta2-adrenergic receptor. Degradation sequences incorporated into the luciferase reporter increase both the rate and relative magnitude of response. Panel A. luc2, firefly luciferase; luc2P, firefly luciferase with PEST sequence (-Pro-Glu-Ser-Thr); luc2CP, firefly luciferase with CL1 and PEST sequences. Panel B. HEK293 cells stably expressing luciferase reporter genes coupled to a cAMP-response element (CRE). Endogenous ß2-adrenergic receptors were induced with 1µM isoproterenol/ 100µM RO-20-1724. |
 |
| Figure 2: Assay strategies using bioluminescence. luc, firefly luciferase gene; Ultra-Glo rLuciferase, a highly stabilised variant of firefly luciferase; P450, cytochrome P450; MAO, monoamine oxidase. |
Another aspect affecting the performance of genetic reporters is response dynamics. A highly stable reporter tends to resist changes in its intracellular concentration, particularly in association with changes in the transcriptional rate. Consequently, the reporter may not accurately represent the transcriptional dynamics in living cells. Dynamic response can be improved by incorporating protein degradation signals into the reporter, but these also reduce intracellular accumulation of the reporter, thus reducing assay sensitivity. This approach works well for luciferase reporters, where the high sensitivity inherent in luminescent assays can compensate for the low intracellular accumulation. In contrast, fluorescent proteins are generally very stable within cells, and addition of degradation signals can substantially restrict assay sensitivity.
The influence of response dynamics is evident in Figure 1, where luciferase expression is coupled to the native ß2-adrenergic receptor in HEK293 cells. Stimulation of this Gas-coupled receptor causes a transient increase in intracellular cAMP, which in turn activates gene expression through a cAMP-response element (CRE). By coupling luciferase to this genetic response element, it thus becomes an intracellular probe of receptor activity. Using the optimised pGL4 Luciferase Reporter Vectors, reporter expression is increased 200-fold following receptor stimulation. The rate and magnitude of this response is improved by adding degradation sequences so that reporter expression more closely follows transcriptional dynamics.
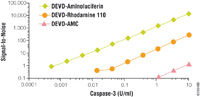 |
| Figure 3: Signal-to-noise ratio comparison of bioluminescent and fluorescent caspase-3/7 assays. |
Detection of ATP is one of the oldest applications for bioluminescence, commonly used for rapid measurement of cell viability. The assay for mammalian cells can be performed in about five minutes and is over 100-fold more sensitive than conventional tetrazolium-based assays. Similar assays for bacterial viability are sensitive to about 100 cells depending on the cell type. Bioluminescent assays for ATP may also be used to measure enzymes that consume ATP, most notably kinases. This provides a nearly universal assay for kinase activity, regardless of whether the phosphate acceptor is a protein, lipid or polysaccharide.
Luciferin may be incorporated into assay designs in a manner similar to fluorogenic assays. By attaching a modifying group, the luciferin becomes unavailable to the luminescent reaction until the modifier is removed through some biochemical process. For example, the luciferin derivative, Asp-Glu-Val-Asp-6'-aminoluciferin (DEVD-Aminoluciferin), cannot support luminescence until the tetrapeptide sequence is cleaved by the caspase-3 protease. Analogous fluorescent assays for apoptosis have been made by coupling the tetra-peptide to appropriate fluorophores such as rhodamine. However, when compared under similar conditions, the bioluminescent assay is nearly 100- to 1,000-fold more sensitive (Figure 3). This strategy can also be used to measure CYP450 activity, monoamine oxidase activity, (Figure 2) or a variety of other enzymatic cleavage reactions.
While rapid protein degradation is advantageous for genetic reporters to increase response dynamics, high enzyme stability is preferred when luciferase is a component of the assay reagent. Thus, for assays based on detection of ATP or luciferin, a modified version of luciferase has been designed for enhanced physical robustness. This stabilised luciferase, called Ultra-Glo rLuciferase, is also resistant to chemical inhibitors such as those found in pharmaceutical compound libraries2.
Bioluminescence has proven to be adaptable for designing rapid, sensitive and simple assays for biochemical- and cell-based assays. Widely popularised for use in genetic reporters, bioluminescence has become routinely applied to a broad range of assay methodologies. These assays are generally recognised for their quantitative precision, low inherent backgrounds, and low sample interference.
References 1. Fan, F. and Wood, K. (2007) Bioluminescent Assays for High-Throughput Screening. Assay Drug Dev. Technol. 5, 127–36. 2. Auld, D.S. et al (2009) A Basis for Reduced Chemical Library Inhibition of Firefly Luciferase Obtained from Directed Evolution. J. Med. Chem. 52, 1450-8.
Author: Keith Wood, Ph.D., Head of Research, Advanced Technologies /Sr. Research Fellow, Promega Corporation










