Let’s get real
24 Jan 2017 by Evoluted New Media
The humble petri dish has been a stalwart of the lab for as long as cells have been cultured – but does 2D cell growth really represent the environment of native tissue?
The humble petri dish has been a stalwart of the lab for as long as cells have been cultured – but does 2D cell growth really represent the environment of native tissue? Cell biology needs to go 3D if it really wants to produce model cultures – here Glauco R. Souza and Thomas C. Killian look at the very cutting edge of this multidimensional approach
2D cell culturing, normally performed on the surface of a common petri dish, has notably improved our understanding of basic cell biology. However, it is becoming increasingly recognised that 2D culture methods do not produce a microenvironment representative of native tissue, which contributes to the observation that tests of drug efficacy and toxicity performed with 2D systems are often not predictive of clinical outcomes. In order to overcome the shortcomings of in vitro 2D cell culture, many 3D cell culturing methods have been developed.
Here, we give an overview of the most common 3D cell culturing products and then focus on a new technology – magnetic 3D cell culturing using 3D bioprinting and/or levitation. Magnetic 3D cell culturing is compatible with a wide range of cell types, including human primary and stem cells, and it can accommodate co-culturing of different cell types and high-throughput architectures. Levitation induces cell-cell interactions and formation of 3D structures more rapidly than other 3D techniques, and the morphology and protein expression of magnetically bioprinted or levitated cells show similarity to in vivo tissue. These results indicate this new and validated technology provides a platform for drug discovery and basic research applications.In order to overcome the shortcomings of in vitro 2D cell culture, many 3D cell culturing methods have been developed
In living tissue, cells exist in 3D microenvironments with intricate cell-cell and cell-matrix interactions and complex transport dynamics for nutrients and cells¹-?. Standard 2D, or monolayer cell cultures are inadequate representations of this environment, which often makes them unreliable predictors of in vivo drug efficacy and toxicity¹,³,?-¹?. Improved in vitro drug screening platforms could translate into significant cost savings for the drug development pipeline and reduction of animal testing. It is now well established that in vitro 3D cell culturing provides a much more physiologically relevant cell-growth environment with great promise for improving basic research and the drug discovery process ¹³, ¹?-²?. However, this approach has not yet been embraced as a routine tool ?,¹?-²¹. Early studies in the 80’s, led by Mina Bissel from the Lawrence Berkeley National Laboratory, highlighted the importance of 3D techniques for creating accurate in vitro culturing models?,²²,²³. This work focused on the importance of the extracellular matrix and the ability of cultures in artificial 3D matrices to produce physiologically relevant multicellular structures, such as acinar structures in healthy and cancerous breast tissue models?,²²-²?. These techniques have proven to be very important for in vitro disease models with a wide range of applications, including evaluating cellular responses to pharmaceutical compounds in drug discovery applications²?.
Improved in vitro drug screening platforms could translate into significant cost savings for the drug development pipeline and reduction of animal testing
Today, there are a large number of culturing tools that claim to provide the advantages of 3D cell culture. The main categories are extracellular matrices or scaffolds, modified surfaces, rotating bioreactors, and microcarriers¹?. A relevant new technology is magnetic 3D cell culture,¹,?-¹?,¹?-¹?,²?,²?, which avoids artificial cell-adhering surfaces. All of these methods claim relative ease of use and produce 3D strucures with appealing properties and improved in vivo similarity compared to 2D methods. However, none of the 3D methods has yet replaced 2D culturing on a large scale, including in the drug development process. This may reflect the relatively recent market availablitly of 3D culture methods, and it is likely that more researchers will adopt 3D methods as the technologies are further validated. However, existing 3D methods are not without limitations, some of which are particulary challenging to overcome in large-scale applications such as drug discovery research. Chief among the limitations is the challenge to integrate 3D culturing techniques with rapid diagnostic tools in order to provide the reproducibility, sensitivity, and speed of high-throughput screening (HTS). Cell based HTS relies on rapid determination of cellular response to drug interaction, such as dose dependent cell viability, cell-cell/cell-matrix interaction, and/or cell migration, but the available assays are not optimised for 3D cell culturing.
The next challenge faced by 3D cell culturing is the limited amount of data/publications that correlate results with in vivo drug response and address mechanisms of drug interaction, cell differentiation, and cell-signaling in in vitro 3D environments. Although the number of 3D cell culturing publications is increasing rapidly, the current amount of biochemical characterization of in vitro 3D tissue does not provide enough confidence to drive large-scale adoption of new methods. Together, the rate at which these challenges are met will determine the pace at which 3D cell culturing is adopted as a routine tool.Today, the most popular 3D culturing platform are still based on artificial matrices and scaffolds that provide a three-dimensional backbone of purified extracellular-matrix (ECM) proteins, bio-compatible polymers, hydrogels, or sponges on which cells can grow. These type of materials and structures allow for more accurate cell-cell interactions and transport properties than 2D systems. The oldest varieties in this category contain large amounts of ECM proteins such as collagen and laminin, which are also believed to recreate important physiologically relevant cues to guide cell differentiation, organisation, and response to external stimuli. An important example is Matrigel (BD), which consists of extracellular material extracted from mouse sarcoma tumor and is prevalent in the breast tissue work mentioned above. A large body of literature exists on culturing in ECM proteins, but there are several well-known concerns²?. The animal origin leads to heterogeneous and often poorly defined molecular composition, with noticeable batch-to-batch variation. This compromises the experimental control and reproducibility.
Additionally, the animal-derived source of the material precludes therapeutic use and may introduce viruses, potentially affecting experimental results. Many of these materials require complex handling procedures such as mixing at ice bath temperatures, tightly controlling pH, special media conditions, and costly growth factors. These protocols can make it difficult to use these products for high-throughput applications. The need for improved cell culturing and the excitement surrounding 3D techniques continues to spawn new research. It would be impossible for us to mention all the projects being pursued in academic and industrial settings, which range from improved matrix materials and spheroid-based approaches, to more innovative ideas like culturing on layered paper sheets. A promising new technology that has recently translated from basic research to commercial availability is magnetic 3D cell culturing through magnetic levitation and 3D bioprinting, marketed by Nano3D Biosciences, and distributed by Greiner Bio-One as the Bio-Assembler.
The need for improved cell culturing and the excitement surrounding 3D techniques continues to spawn new research
It is designed to provide the advantages of 3D cell culturing in a simpler platform that can be incorporated into existing protocols and diagnostics and adapted for high-throughput applications. The Bio-Assembler uses biopolymer-based Nanoshuttle reagents to deliver magnetic nanoparticles to individual cells so that an applied magnetic drive can aggregate them by levitation or bioprinting to initiate and facilitate cell-cell interactions in the absence of any artificial surface or matrix (Figure 1). Magnetic forces can also be used to bioprint tissue in a large array of patterns. The magnetic fields are designed to rapidly form 3D multicellular structures in as little as a few hours, including expression of ECM proteins, which is much faster than other 3D cell culturing techniques. There is no need for additional equipment or special media or growth factors, and the ability to manipulate cells and shape tissue magnetically offers new possibilities for controlled co-culturing of different cell types and for development of new cell migration/invasion assays and wound-healing models¹,?-?.
[caption id="attachment_57372" align="alignnone" width="620"]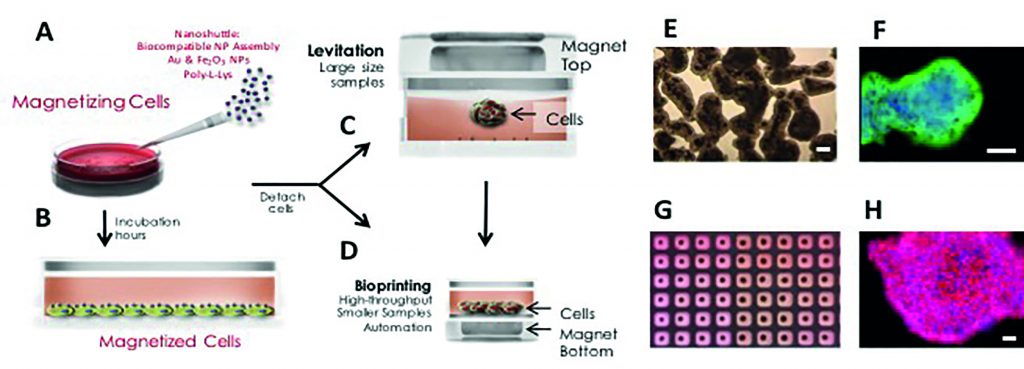 Magnetic 3D cell culturing. (A) Nanoshuttle is disbursed over the cells, (B) which are then incubated for several hours. Cells are detached and transferred to a new tissue culture plate and then (C) cultured in 3D by magnetic levitation or (D) magnetically 3D bioprinted. (C, E, F) For 3D-cell culturing by magnetic levitation, the drive is placed on top of the tissue culture dish, and cells are magnetically lifted off the bottom. (D, G, H) For magnetic 3D bioprinting, a patterned magnetic drive is placed at the bottom of a cell-repellent tissue-culture plate. Cells rapidly assemble (minutes) into a shape mirroring the shape of the magnets, typically arrays of rings or a dots. Cell-repellent tissue-culture plate is required to prevent cells from adhering and growing as a monolayer in 2D. (E) Bright-field micrograph of levitating human primary fibroblasts after 12 hours of levitation. (F) Magnetically levitated 3D culture of human primary bronchial epithelial cells levitated for 48 hours, with immunohistochemical staining for E-Cadherin (green; nuclei were counterstained with DAPI, blue). (G) Photograph of bioprinted spheroids in 384-well plate. (H) Immunohistochemical staining of a magnetically bioprinted spheroid of mouse 3T3 fibroblasts for fibronectin (red; nuclei were counterstained with DAPI, blue). All scale bars are 50 ?m.[/caption]
Magnetic 3D cell culturing. (A) Nanoshuttle is disbursed over the cells, (B) which are then incubated for several hours. Cells are detached and transferred to a new tissue culture plate and then (C) cultured in 3D by magnetic levitation or (D) magnetically 3D bioprinted. (C, E, F) For 3D-cell culturing by magnetic levitation, the drive is placed on top of the tissue culture dish, and cells are magnetically lifted off the bottom. (D, G, H) For magnetic 3D bioprinting, a patterned magnetic drive is placed at the bottom of a cell-repellent tissue-culture plate. Cells rapidly assemble (minutes) into a shape mirroring the shape of the magnets, typically arrays of rings or a dots. Cell-repellent tissue-culture plate is required to prevent cells from adhering and growing as a monolayer in 2D. (E) Bright-field micrograph of levitating human primary fibroblasts after 12 hours of levitation. (F) Magnetically levitated 3D culture of human primary bronchial epithelial cells levitated for 48 hours, with immunohistochemical staining for E-Cadherin (green; nuclei were counterstained with DAPI, blue). (G) Photograph of bioprinted spheroids in 384-well plate. (H) Immunohistochemical staining of a magnetically bioprinted spheroid of mouse 3T3 fibroblasts for fibronectin (red; nuclei were counterstained with DAPI, blue). All scale bars are 50 ?m.[/caption]
Recently, n3D in collaboration with AstraZeneca published in Scientific Reports? the methodology for a vasoactivity high-throughput assay, which demonstrates how magnetically 3D bioprinted rings of vascular smooth muscle cells can structurally represent a blood vessel segment and its contractibility can be used to measure compound vasoactivity (Figure 2)?. This assay not only addresses the lack of in vitro and high-throughput tools to screen vasoactivity but also the need for a surrogate to the current method for screening compound vasoactivity, the ex vivo aorta ring assay. This ex vivo assay uses blood vessels from animals, which is not only a poor representation of human blood vessel physiology, it is also low-throughput, requires a large number of animals, and is costly. As next phase of this work, we are incorporating the co-culture of endothelial cells along with smooth muscle for fine tuning the physiology of vasodilation and vasoconstriction of human blood vessels.
[caption id="attachment_57373" align="alignnone" width="620"]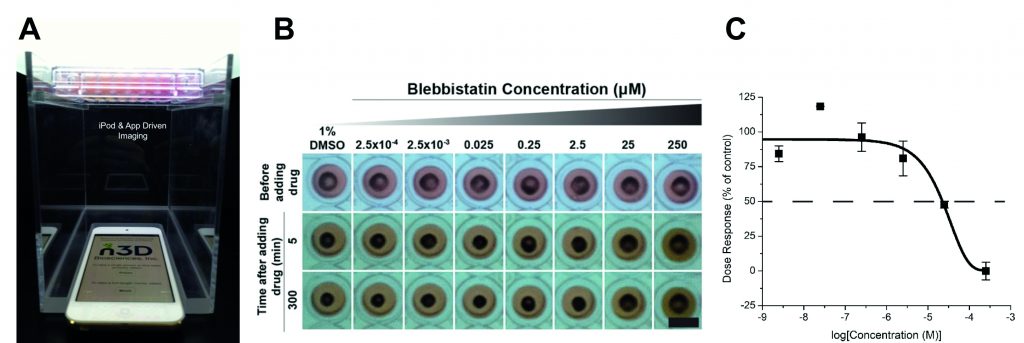 Figure 2: (A) The mobile device-based imaging system,7–9 with magnetically 3D bioprinted cells placed above the mobile device, which can be set to image the entire plate at programmed intervals as short as 1?s. (B) Magnetically 3D bioprinted rings of vascular smooth muscle taken with the mobile device-based imaging system with varying concentrations of vasodialating compound blebbistatin; rings exposed to higher compound concentrations are unable to contract as fast as control; scale bar is 5?mm.8 (C) Representative dose-response curve of measurements shown in (B)7–9.[/caption]
Figure 2: (A) The mobile device-based imaging system,7–9 with magnetically 3D bioprinted cells placed above the mobile device, which can be set to image the entire plate at programmed intervals as short as 1?s. (B) Magnetically 3D bioprinted rings of vascular smooth muscle taken with the mobile device-based imaging system with varying concentrations of vasodialating compound blebbistatin; rings exposed to higher compound concentrations are unable to contract as fast as control; scale bar is 5?mm.8 (C) Representative dose-response curve of measurements shown in (B)7–9.[/caption]
Figure 1 describes the magnetic 3D cell culture method, which is compatible with standard diagnostics such in situ imaging, fixing, and immunohistochemistry, and is not significantly more complicated than standard 2D culturing methods. Key biological observations of magnetically bioprinted or levitated cells (morphology, extracellular matrix formation, protein expression, and function) and significant practical advantages of the technique (especially speed to form 3D structures and ease of manipulation) show the promise of this technology for various areas of biomedical research and industry¹,?,?,¹?-¹?,²?.
All cell types tested with magnetic 3D cell culturing have been cultured successfully, including human cell lines (glioblastomas, normal astrocytes, HEK 293, breast cancerMDA-231, breast MCF10A, 3T3-fibrolast - pre-adipocytes, hepatoma H-4-II-E, bone marrow endothelial cells, melanoma, Kaposi’s sarcoma),¹,? stem cells (murine neural stem cells, mesenchymal stem cells, and dental pulp stem cells),¹,¹?,¹?and primary cells (endothelial, smooth muscle, epithelial, fibroblasts, chondrocytes, and cells isolated from adipose tissue stromal vascular fraction)?,?Morphology is a good baseline metric for cell cultures, especially for assessing in vivo similarity¹?. The morphology observed in levitated tissue is strikingly similar to that seen in vivo, which can be attributed to the absence of a perturbing plastic surface and the ability of cells to interact and expand in a more natural 3D microenvironment of other cells and signals. Human small airway epithelial cells (Figure 1F) spontaneously assemble with a phenotype that is characteristic of E-Cadherin expression at the cell-cell interface. Mammary epithelial cells grown with magnetic levitation develop characteristic acinar structures, which are difficult to achieve in 2D. Such structures are indicative of normal cell polarization and growth arrest and can allow one to distinguish between normal and tumorigenic cells. Results are very similar to observations in the current 3D culture standard -reconstituted basement membrane (Matrigel).
All cell types cultured to date with magnetic levitation form strong, cohesive tissue structures in less than 24 hrs, which suggests that components of endogenous extracellular matrix are being produced. This is supported by experiments with primary human pulmonary fibroblast (Figure 1E) and tracheal smooth muscle that show a much higher level of laminin in cells grown with magnetic levitation compared to 2D cell cultures. A fully competent extracellular matrix in in vitro culture is important for accurate prediction of clinical efficacy/toxicity, and these results are encouraging.Protein expression in magnetically bioprinted or levitated cultures also displays a strong similarity to in vivo patterns. N-cadherin expression in levitated human glioblastoma cells was identical to the expression seen in human tumor xenografts grown in immune deficient mice,¹ while standard 2D culture showed much weaker expression that did not match xenograft distribution. The transmembrane protein N-cadherin is often used as an indicator of in-vivo-like tissue assembly in 3D culturing¹,?There are also indications that in vivo cell function is recapitulated in levitated tissue. In levitated cultures, the invasiveness of glioblastoma cells in normal human astrocytes matched patterns seen in xenograft, while other in vitro culturing methods, including 3D culturing in BM (Matrigel), failed to reproduce this behaviour¹,²?.
For magnetic 3D cell culturing, cells must uptake magnetic nanoparticles, and the effects of the nanoparticles on biological function is an obvious question. Rigorous tests have consistently confirmed the cytocompatibility and bioinertness of the nanoparticle assembly. The particles used are biocompatible and commonly used in many biological applications, no deleterious effects of nanoparticles have been observed in any of the cell types cultured, and do not interfere with fluorescence or other assays. Previous studies have shown that neither Nanoshuttle nor the magnetic field have any effect on proliferation, metabolism?,?,¹?,¹16, and viability¹?, and will not create inflammatory¹? or oxidative stress¹?. Cells remain healthy and continue to expand literally for months, stopping only at cessation of the experiment. In fact, some cells have been shown to grow faster in 3D than in 2D¹1.TEM images of glioblastoma cells showed that cells expel nanoparticles into the surrounding extracellular matrix within a few days1. Comparative genomic hybridization (CGH) screening of human pulmonary fibroblast cells showed no chromosomal abnormalities in cells treated with nanoparticles and levitated. The application of forces to cells, as studied in mechano-biology, can up-regulate protein expression and drive cellular differentiation, but the typical levitation force on an individual cell is ~10 pN, which is much smaller than the forces cells exert on each other through the extracellular matrix during movement or tissue reconstruction.As 3D cell culturing techniques become more developed and widely prevalent, they will undoubtedly play an important role in drug discovery and basic biological research. A wide assortment of different platforms is now commercially available, and it is likely that many of these technologies will prove valuable for different applications. Magnetic levitation is a relative newcomer among 3D technologies, but has several advantages over existing methods that make it a promising technology for life science applications.

 Thomas C. Killian is a Professor of Physics and Astronomy at Rice University. His research interests include techniques to manipulate biological structures with EM fields. He is a co-founder of Nano3D Biosciences
Thomas C. Killian is a Professor of Physics and Astronomy at Rice University. His research interests include techniques to manipulate biological structures with EM fields. He is a co-founder of Nano3D Biosciences
References
- Souza, G. R. et al. Three-dimensional tissue culture based on magnetic cell levitation. Nat. Nanotechnol. 5, 291–296 (2010).
- Ravi, M., Paramesh, V., Kaviya, S. R., Anuradha, E. & Paul Solomon, F. D. 3D cell culture systems: Advantages and applications. J. Cell. Physiol. 230, 16–26 (2015).
- Edmondson, R., Broglie, J. J., Adcock, A. F. & Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol. 12, 207–18 (2014).
- Lee, G. Y., Kenny, P. A., Lee, E. H. & Bissell, M. J. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat. Methods 4, 359–365 (2007).
- Pampaloni, F. & Stelzer, E. H. K. Three-Dimensional Cell Cultures in Toxicology. Biotechnol. Genet. Eng. Rev. 26, 129–150 (2009).
- Marx, V. Cell culture: a better brew. Nature 496, 253–258 (2013).
- Timm, D. M. et al. A high-throughput three-dimensional cell migration assay for toxicity screening with mobile device-based macroscopic image analysis. Sci. Rep. 3, 3000 (2013).
- Tseng, H. et al. A high-throughput in vitro ring assay for vasoactivity using magnetic 3D bioprinting. Sci. Rep. 6, 30640 (2016).
- Tseng, H. et al. A spheroid toxicity assay using magnetic 3D bioprinting and real-time mobile device-based imaging. Sci. Rep. 5, 13987 (2015).
- Becker, J. L. & Souza, G. R. Using space-based investigations to inform cancer research on Earth. Nat. Rev. Cancer 13, 315–327 (2013).
- Pampaloni, F., Reynaud, E. G. & Stelzer, E. H. K. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 8, 839–845 (2007).
- Boudreau, N. & Weaver, V. Forcing the Third Dimension. Cell 125, 429–431 (2006).
- Griffith, L. G. & Swartz, M. A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 7, 211–224 (2006).
- Haisler, W. L. et al. Three-dimensional cell culturing by magnetic levitation. Nat. Protoc. 8, 1940–1949 (2013).
- Daquinag, A. C., Souza, G. R. & Kolonin, M. G. Adipose tissue engineering in three-dimensional levitation tissue culture system based on magnetic nanoparticles. Tissue Eng. Part C. Methods 19, 336–344 (2013).
- Tseng, H. et al. Assembly of a three-dimensional multitype bronchiole coculture model using magnetic levitation. Tissue Eng. Part C. Methods 19, 665–675 (2013).
- Tseng, H. et al. A three-dimensional co-culture model of the aortic valve using magnetic levitation. Acta Biomater. 10, In press (2013).
- Decaestecker, C., Debeir, O., Van Ham, P. & Kiss, R. Can anti-migratory drugs be screened in vitro? A review of 2D and 3D assays for the quantitative analysis of cell migration. Med. Res. Rev. 27, 149–176 (2007).
- Justice, B. A., Badr, N. A. & Felder, R. A. 3D cell culture opens new dimensions in cell-based assays. Drug Discov. Today 14, 102–107 (2009).
- Prestwich, G. D. Evaluating drug efficacy and toxicology in three dimensions: using synthetic extracellular matrices in drug discovery. Acc. Chem. Res. 41, 139–148 (2008).
- Lai, Y., Asthana, A. & Kisaalita, W. S. Biomarkers for simplifying HTS 3D cell culture platforms for drug discovery: the case for cytokines. Drug Discov. Today (2011).
- Lee, E. Y., Lee, W. H., Kaetzel, C. S., Parry, G. & Bissell, M. J. Interaction of mouse mammary epithelial cells with collagen substrata: regulation of casein gene expression and secretion. Proc. Natl. Acad. Sci. U. S. A. 82, 1419–1423 (1985).
- Petersen, O. W., Ronnov-Jessen, L., Howlett, A. R. & Bissell, M. J. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc. Natl. Acad. Sci. U. S. A. 89, 9064–9068 (1992).
- Leonard, F. & Godin, B. in Breast Cancer: Methods and Protocols (ed. Cao, J.) 239–251 (Springer New York, 2016). doi:10.1007/978-1-4939-3444-7_21
- Han, J. et al. Molecular Predictors of 3D Morphogenesis by Breast Cancer Cell Lines in 3D Culture. PloS Comput. Biol. 6, e1000684 (2010).
- Hogan, M., Souza, G. & Birla, R. Assembly of a functional 3D primary cardiac construct using magnetic levitation. AIMS Bioeng. 3, 277–288 (2016).
- Tseng, H. et al. Luminescent Viability Assays in Magnetically Bioprinted 3D Cultures Limitations of Two-Dimensional Cell Culture Models. Promega PubHub 9/15, (2015).
- Polykandriotis, E., Arkudas, A., Horch, R. E. & Kneser, U. To matrigel or not to matrigel. Am. J. Pathol. 172, 1441; author reply 1441--2 (2008).
- Molina, J. R., Hayashi, Y., Stephens, C. & Georgescu, M.-M. M.-M. Invasive glioblastoma cells acquire stemness and increased Akt activation. Neoplasia 12, 453–463 (2010).











